

He reacted silver cyanate (AgOCN) and ammonium chloride (NH 4Cl), expecting to get ammonium cyanate (NH 4OCN). The vital force theory began to decline in 1828, when the German chemist Friedrich Wöhler synthesized urea from inorganic starting materials. For many years, scientists thought organic compounds could be made by only living organisms because they possessed a vital force found only in living systems. Compounds isolated from nonliving systems, such as rocks and ores, the atmosphere, and the oceans, were labeled inorganic. Scientists of the 18th and early 19th centuries studied compounds obtained from plants and animals and labeled them organic because they were isolated from “organized” (living) systems. To be able to recognize the composition and properties typical of organic and inorganic compounds.Chapter 10 focuses on organic acids and bases. Then in Chapter 9, we will study some compounds considered to be derived from hydrocarbons by replacing one or more hydrogen atoms with an oxygen-containing group. In Chapter 8 we will examine hydrocarbons with double bonds, with triple bonds, and with a special kind of bonding called aromaticity.
There are several other kinds of hydrocarbons, distinguished by the types of bonding between carbon atoms and by the properties that result from that bonding. Recall that halogens are the elements in Family 7A on the periodic table and contain representative elements such as chlorine, fluorine, iodine, and bromine. We will also investigate alkanes that have halogens incorporated into their structure. In this chapter we will investigate the alkanes, compounds containing only two elements, carbon and hydrogen, and having only single bonds.
#Alkane functional group skin
Water and aqueous solutions such as urine will not dissolve such a film, which explains why petroleum jelly protects a baby’s tender skin from diaper rash. Such alkane mixtures as mineral oil and petroleum jelly can be applied as a protective film. Liquid alkanes with approximately 5–16 carbon atoms per molecule wash away natural skin oils and cause drying and chapping of the skin, while heavier liquid alkanes (those with approximately 17 or more carbon atoms per molecule) act as emollients (skin softeners). (There is no home-treatment antidote for gasoline poisoning call a poison control center.) People who swallow gasoline or other liquid alkane mixtures should not be made to vomit, as this would increase the chance of getting alkanes into the lungs. The lungs become unable to expel fluids, just as in pneumonia caused by bacteria or viruses. In the lungs, however, they cause “chemical” pneumonia by dissolving fatlike molecules from cell membranes in the tiny air sacs (alveoli). Swallowed, liquid alkanes do little harm while in the stomach. A range of petroleum-based products that can be abused as inhalants. Pressurized canisters of propane and butane gas, both of which are intended for use as fuels, are abused as inhalants.įigure 7.1. Inhaling (“sniffing”) these hydrocarbons in gasoline or aerosol propellants for their intoxicating effect is a major health problem that can lead to liver, kidney, or brain damage or to immediate death by asphyxiation by excluding oxygen. Alkanes of low molar mass-those with from 1 to approximately 10 or so carbon atoms-are gases or light liquids that act as anesthetics. These effects depend on the size of the hydrocarbon molecules and where on or in the body they are applied.
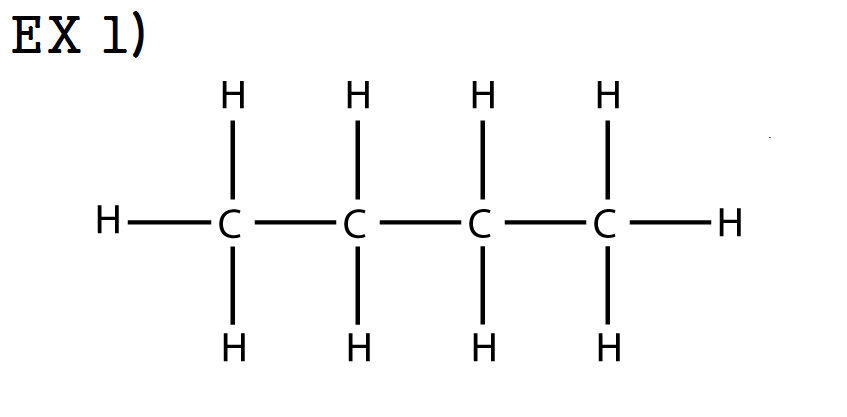
Hydrocarbons are the simplest organic compounds, but they have interesting physiological effects.


 0 kommentar(er)
0 kommentar(er)
